祝贺我团队司璐颖、牟方志、马会茹等撰写的论文“Swarming Magnetic Fe3O4@Polydopamine-Tannic Acid Nanorobots: Integrating Antibiotic-Free Superficial Photothermal and Deep Chemical Strategies for Targeted Bacterial Elimination”被期刊Research(2023年IF:8.5,中科院一区期刊)接收!
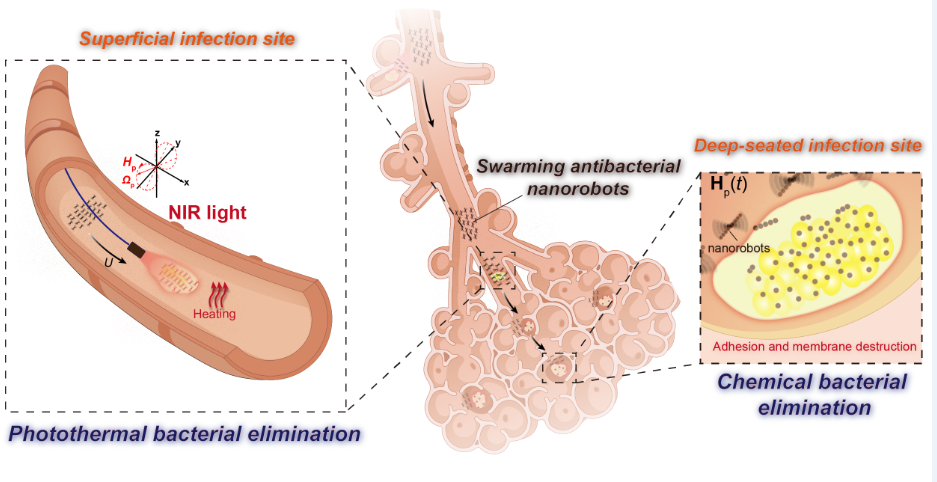
微纳机器人 (MNRs) 有望通过将各种抗菌剂或能量输送到许多难以到达的感染部位,从而为感染性疾病治疗带来革命性的变化。然而,现有 MNRs 在应对从浅表到深层组织的复杂感染方面面临巨大挑战。为此,我们研制集成了浅表光热和深层化学抗菌策略的磁驱Fe3O4@聚多巴胺-单宁酸纳米机器人(Fe3O4@PDA-TA NRs)集群,实现了对复杂感染病灶区域的靶向抗菌。通过DA表面原位聚合和TA吸附改性两步法可以简易制备得到纳米机器人基元Fe3O4@PDA-TA纳米粒子(NPs)。在交变磁场的驱动下,这些 NPs能够组装形成具有强大驱动力的磁驱NRs群体,并展现出可控的集群运动。它们能从远端导航至浅表病灶并快速大面积覆盖目标区,进而在近红外(NIR)光照射下实现快速光热灭菌。而且,这种集群机器人还可以穿越狭窄曲折通道并富集于深层感染部位,借助TA对细菌表面的粘附和对细胞膜的破坏实施持续化学抗菌作用。本文合成的Fe3O4@PDA-TA NRs集群在包含浅表和深层组织的复杂感染疾病治疗方面表现出巨大潜力。该研究将对未来多功能协同、灵活、高效的疾病治疗微系统的发展提供启示。
原文摘要如下:Micro/nanorobots (MNRs) are envisioned to provide revolutionary changes to therapies for infectious diseases as they can deliver various antibacterial agents or energies to many hard-to-reach infection sites. However, existing MNRs face significant challenges in addressing complex infections that progress from superficial to deep tissues. Here we develop swarming magnetic Fe3O4@polydopamine-tannic acid nanorobots (Fe3O4@PDA-TA NRs) capable of performing targeted bacteria elimination in complicated bacterial infections by integrating superficial photothermal and deep chemical strategies. The Fe3O4@PDA-TA nanoparticles (NPs), serving as building blocks of the nanorobots, are fabricated by in-situ polymerization of dopamine followed by TA adhesion. When driven by alternating magnetic fields, the Fe3O4@PDA-TA NPs can assemble into large energetic microswarms continuously flowing forward with tunable velocity. Thus, the swarming Fe3O4@PDA-TA NRs can be navigated to achieve rapid broad coverage of a targeted superficial area from a distance, and rapidly eradicate bacteria residing there upon exposure to near-infrared (NIR) light due to their efficient photothermal conversion. Additionally, they can concentrate at deep infection sites by traversing through confined, narrow, and tortuous passages, exerting sustained antibacterial action through their surface TA-induced easy cell adhesion and subsequent membrane destruction. Therefore, the swarming Fe3O4@PDA-TA NRs show great potential for addressing complex superficial-to-deep infections. This study may inspire the development of future therapeutic microsystems for various diseases with multifunction synergies, task flexibility, and high efficiency.